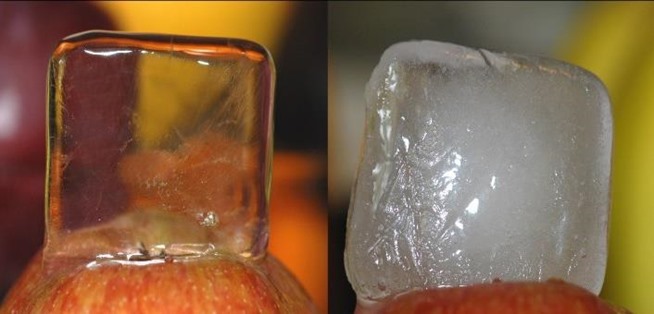
A common trick to make clear water ice is to boil pure water prior to freezing it. Why does that work and what are the white inclusions in ice that was made from unboiled tap water?
Thermodynamics – Why is Ice Made from Boiled Water Clear?
everyday-lifefreezingicethermodynamicswater
Best Answer
The short answer: Cloudy ice is caused by gases (mainly nitrogen and oxygen) dissolved in the water that come out of solution when the water freezes. The small bubbles trapped in the ice cause the white appearance. Boiling the water removes the air dissolved in it, producing clear ice as a result. Assuming that other impurities don't produce the same cloudy effect.
The long answer:
Impurities present in water:
Gases: Water at 20°C normally contains about 15 ppm dissolved gases, which is the equivalent of 1 volume of air per 50 volumes of water. These are the same gases present in air, but not in the same proportions since some are more soluble than others: it's about 63% nitrogen, 34% oxygen, 1.5% argon and 1.5% carbon dioxide.
minerals: Tap water contains dissolved minerals, mainly Ca and Mg. They can be present in the form of bicarbonates: $Ca^{2+}({HCO_3}^-)_2$ and $Mg^{2+}({HCO_3}^-)_2$ (these only exist in solution, not as solid substances), and as calcium and magnesium sulphate. If the water passed through a water softener, the Ca and Mg ions may have been replaced by (twice as many) sodium or potassium ions.
The effects of heating the water:
removing dissolved gases: higher temperature favors endothermic reactions (Le Chatelier's principle). For the gases present in water, dissolution (at room temperature) is an exothermic process, so their solubility decreases when the water is heated. The solubility of gases doesn't reach zero at boiling point, nor does it necessarily decrease over the whole temperature range. For nitrogen in water, the enthalpy of dissolution becomes positive around 75°, and its solubility increases above that temperature. At 100°C, solubility of air as a whole is $0.93 * 10^{-5}$, about half the solubility at 10°C, $1.82 * 10^{-5}$.
removing dissolved minerals: Heating the water promotes the conversion of soluble Ca and Mg bicarbonates to insoluble carbonates ($2 {HCO_3}^-$ → $CO_3^{2-} + H_2O + CO_2$) which will come out of solution (as limescale). The sulphates (sometimes referred to as "permanent hardness"), and the sodium or potassium (bi)carbonates stay in solution.
The effect of boiling:
How do gases make ice "milky/cloudy"?